
Constituents in water
L.A. Baker, B.C. Asleson, A.J. Erickson, and R.M. Hozalski
There are many constituents in stormwater runoff that may be of concern for an assessment program, several of which are discussed below. Within each group, the appropriate analysis may depend on the assessment goals, available analytical equipment, analysis cost, and potential for in situ measurement.
Suspended solids
Analysis for total suspended solids (TSS) is based on filtration of a subsample withdrawn from a larger sample bottle through a glass fiber filter (A.P.H.A. 1998b). This technique may be inaccurate when samples contain a significant amount of sand-sized particles ( > 0.062 microns) because these particles settle quickly and therefore it is challenging to obtain a representative subsample (Gray et al. 2000, Selbig et al. 2007). Gray et al. (2000) claim that the TSS analysis method is “fundamentally unreliable” for the analysis of natural water samples and recommend measuring suspended-solids concentration (SSC).
A.S.T.M. (2000) includes three analysis methods for determining SSC. For samples with large suspended concentrations, the wet-sieving method (method C) is recommended. This method involves wet sieving the entire sample through a 62 micron (0.002 inches) sieve followed by filtration through glass fiber filter. In addition to providing better measurement of SSC, this method also provides some information on the distribution of particle size diameter.
For detailed analysis of particle retention, more detailed information on the particle size distribution in stormwater can be obtained by sieving followed by analysis using the hydrometer method (A.S.T.M. 2000), which yields information on silt and clay-sized particles. Analysis of volatile suspended solids (VSS) can be used to estimate the contribution of organic matter to TSS.
Salinity-related variables
Salinity is generally defined as the total dissolved solids (TDS), which is the mass concentration (mg/L) of all ions in solution. In practice, eight ions comprise nearly all of TDS (A.P.H.A. 1998b), as given in equation 6.1.
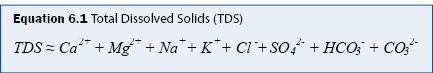
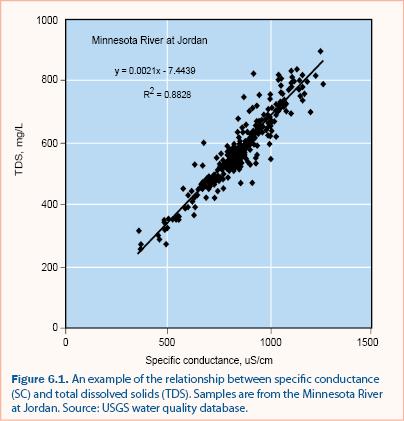
It is often unnecessary to measure all major ions for a stormwater assessment program. Gravimetric analysis for measuring TDS is tedious and therefore it is common to estimate TDS from measurements of specific conductance (SC), which can be measured in situ. A regression relationship between TDS and SC must be developed from at least 25 samples collected over a range of TDS and SC values (e.g., see figure 6.1). Once the relationship is determined, SC is measured directly, and the SC-TDS regression relationship is used to estimate TDS. The SC-TDS relationship may not be valid if the ionic composition of the water changes significantly, as may occur with road salt deicer application in cold climates. It is recommended that the SC-TDS regression be verified regularly and that multiple regressions be developed if there are significant differences between different time periods (e.g., winter months vs. summer months).
Natural organic matter
In addition to VSS (above), two common metrics of organic matter in water include biochemical oxygen demand (BOD) and chemical oxygen demand (COD). BOD is a measure of readily decomposable organic matter, generally measured over a period of five days (BOD5). Measurements of BOD are needed when an assessment goal is to determine the reduction of oxygen-depleting material by a stormwater treatment practice. COD measurements are often less expensive and it is common practice to estimate BOD from COD in wastewater because the relationship between BOD and COD is fairly constant at ~2:1 (Metcalf and Eddy 1991). However, this is not advisable for stormwater because the ratio varies significantly (Maestre and Pitt 2005).
Phosphorus
Phosphorus (P) exists in many forms in the environment: as phosphate ions, as polyphosphates, as a component of RNA and DNA, and in phospholipids. Analysis of the exact chemical form of P requires specialized analytical techniques that are generally used only in research projects. For stormwater assessment, total P is commonly separated into particulate and dissolved by filtering through a 0.45 μm filter. Sometimes, dissolved P is separated into total dissolved and reactive dissolved (also called soluble reactive) although it is more common to measure total P and reactive dissolved P due to the additional cost of measuring total dissolved P. Distinction between these forms of P is based on operational definitions of analysis including filtration and type of digestion (figure 6.2).
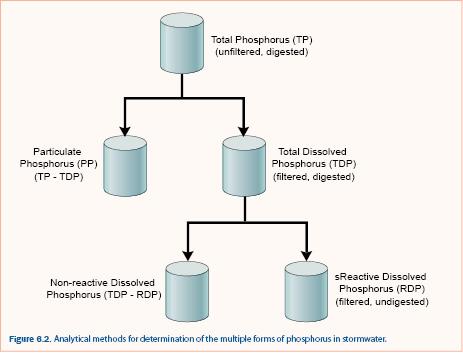
Total P is determined by using one of several strong acid digestion techniques on an unfiltered sample and measuring P concentration, typically by a colorimetric method. Dissolved reactive P is determined by measuring the phosphorus concentration of a filtered sample (filtrate). Filtered samples may be digested to determine total dissolved P. Particulate phosphorus and nonreactive dissolved phosphorus are calculated by difference as shown in figure 6.2. Knowledge of the different forms of P is important because P removal processes are dependent upon the form. For example, sedimentation or filtration can typically capture particulate P but not dissolved P. It is also possible that transformation of P occurs within stormwater treatment practices, which can only be determined if both particulate and dissolved phosphorus fractions are measured.
Nitrogen
Similar to phosphorus, total nitrogen is separated in several common forms of nitrogen (N) which are typically determined operationally by filtration, digestion, and chemical analysis. The most common forms of N in stormwater include particulate N, dissolved organic N (DON), nitrate (NO3-), nitrite (NO2-) and ammonium (NH4+). There is no single method for analyzing total N and therefore it is common for samples to be digested using Kjeldahl digestion, which converts organic N (particulate N and DON) to ammonium. The ammonium concentration is then measured by one of several analysis methods (A.P.H.A. 1998b) and the resulting concentration is called total Kjeldahl nitrogen (TKN) which includes organic N plus the ammonium that existed in the sample before digestion. Nitrate (NO3-) and nitrite (NO2-) can be measured individually and total N can be estimated as the sum of TKN + (NO3-) + (NO2-).
Algae abundance
Planktonic algae (algae suspended in water) are a form of suspended solids but do not behave like inorganic particles. Algae grow within a pond, essentially forming “new” suspended particles, as opposed to particles from the contributing watershed. In addition, algae do not settle like inorganic particles because they have lower density and different shapes. Large concentrations of algae, particularly blue-green algae, can become a nuisance and health hazard (primarily to pets) for homeowners living near a wet pond or downstream receiving water bodies.
Chlorophyll concentration provides an accurate measure of total algal abundance, and an estimate of blue-green algae. For most stormwater applications, chlorophyll samples are collected and then filtered. The chlorophyll on the filter is then extracted into acetone or another solvent and measured by spectrophotometry or fluorometry (A.P.H.A. 1998b). When samples cannot be analyzed immediately, filters are frozen for preservation. Chlorophyll can also be measured in situ using fluorescence-based monitors. This enables real-time continuous measurement, which could be useful for assessment programs that focus on algae.
As a rule of thumb, chlorophyll comprises approximately 1% of algae (dry weight basis). Therefore a sample with 0.1 mg chlorophyll/L (a hypereutrophic pond) is equivalent to a TSS concentration of approximately 10 mg/L.
Metals
Urban stormwater often contains metals at levels of environmental significance, including cadmium, zinc, copper, and lead (see Stormwater Treatment Processes). Metals in water are most commonly measured as “total” concentration (unfiltered samples) and “dissolved” concentration (filtered samples, generally through a 0.45 μm filter). Metals must be in the dissolved state to be biologically available, so measuring both dissolved and total metal concentrations is advised. Samples for “total” metals analyses are digested with strong acids and/or oxidants to dissolve metals that are particles or bound to particles. Digestion techniques vary with respect to completeness in releasing metals from solution, so the description of “total” is operationally defined by the type of digestion used (A.P.H.A. 1998b). The most rigorous digestions may involve hazardous materials (such as hydrofluoric acid or perchloric acid), so unless this level of digestion is required, milder, safer digestion procedures are generally used. The analytical laboratory involved in the assessment project should be consulted regarding digestion procedures.
Once in soluble form, the metals are measured by atomic adsorption spectrometry or inductively coupled plasma (ICP) emission spectrometry. Atomic adsorption measures one element at a time but generally has lower detection limits while ICP can be used to measure several different metals simultaneously.
Metals readily bind to soils but do not degrade; therefore accumulation of metals over time is expected in infiltration and filtration practices. Using typical values of stormwater loading and soil capacity estimates, Davis et al. (2003) estimated that, after 20 years, concentrations of cadmium, lead, and zinc would reach or exceed levels permitted by U.S. EPA biosolids land application regulations while Legret et al. (1999) conducted studies and applied mathematical models to determine that the increase in soil metal concentrations would be slight after 50 years. Studies have found that metals are typically retained on the soil particles within the first 5 to 15 inches with cadmium having the greatest downward mobility (Barraud et al. 1999, Dierkes and Gieger 1999).
Some heavy metals such as zinc and copper are also micronutrients used by plants and may be accumulated into plant biomass as the plant grows. Plant species accumulate heavy metals at different rates and have different metal tolerances (Sun and Davis 2007). There may therefore be a need to measure changes in the metal content of soils over time. Further discussion, including analysis methods, are discussed in the following section.
Continue to Constituents in soils.